The standard conditioning regimen for multiple myeloma patients undergoing autologous hematopoietic stem cell transplant (HSCT) is intravenous melphalan dosed at 200mg/m 2 (mel200). Prior retrospective studies indicated that dose reduction to melphalan 140 mg/m 2 (mel140) confers similar progression-free (PFS) and overall survival (OS), leading to increased use of mel140 in patients with comorbidities or advanced age. A recent single-center retrospective study (Sharma et al, Leukemia Lymphoma 2023) identified an OS advantage yet similar PFS for mel200 vs mel140, suggesting that certain patient cohorts may benefit from mel200. These findings require confirmation and better understanding of the risks and benefits to different dosing strategies.
We performed a single-center retrospective study of mel dosing and transplant outcomes at the University of Nebraska Medical Center between 2008-2018. Three hundred and sixty-two patients underwent a first autologous HSCT with mel conditioning; 95 received mel140 while 267 received mel200. Nearly all patients received maintenance therapy (n= 357, 99%).
Patient demographic and disease factors were largely comparable between mel140 and mel200 cohorts including sex, response prior to HSCT (both 73% for very good partial response or better), and presence of high-risk cytogenetics (t(4;14), t(14;16), t(14;20), del17p; 15% vs 14%). The mel140 group was older (65.1 years vs 59.7 years), had higher hematopoietic cell transplantation-specific comorbidity index (HCT-CI) scores (64% vs 40% at ³3), and International Staging System (ISS) stages (40% vs 22% at stage III) than the mel200 group. Average creatinine clearance at transplant was 71 vs 91 mL/min/m 2 in the mel140 vs mel200 cohorts, respectively.
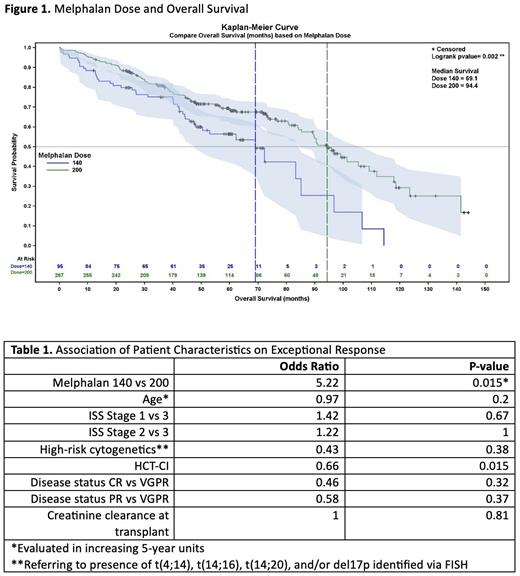
OS favored mel200 with a median survival time of 94.4 vs 69.1 months (mel200 vs mel140, Figure 1, p = 0.002). There was no statistical difference in PFS between mel200 vs mel140 (median PFS 73.0 vs 60.5 months, p = 0.43, respectively). With Cox proportional hazards regression, we performed a multivariate analysis of risk factors on PFS and OS. For OS, age ( p = 0.02) retained statistical significance within the model. Mel dosing and high-risk cytogenetics (both p = 0.09) did not retain significance. HCT-CI may have an effect ( p = 0.04), but due to missing data on n= 125/362 subjects this could not be well evaluated. Response prior to HSCT, creatine clearance at transplant, and ISS stage were not statistically significant.
Given the multiple variables assessed and missing data for HCT-CI we separately analyzed mel dosing together with the most significant variable in the model, age. In this subset OS model, mel200 was associated with an improved OS (hazard ratio (HR) 0.64, p = 0.03) while age was associated with an adverse effect (HR 1.11 per 5-year increase, p = 0.06). For the multivariate PFS model, only high-risk cytogenetics (HR 1.82 yes vs no, p = 0.004) retained statistical significance. Median engraftment was faster in mel200 cohort (67% vs 92% engraftment starting at ³D+12, p <0.001).
We next examined the impact of mel dosing on the rate of exceptional response (Paquin et al, Blood Cancer Journal 2020), defined as alive and progression-free for 8 or more years. Twenty-nine of 362 patients (8%) met the requirements for exceptional response. Two (7%) received mel140 while 27 (93%) received mel200. Exceptional responders were younger and split evenly between male and female. The majority had at least a very good partial response prior to HSCT (79%). Only 2 exhibited high-risk cytogenetics; one received bortezomib and lenalidomide maintenance, the other only bortezomib.
On multivariate analysis, only two factors contributed to exceptional response: mel dose (mel200 odds ratio (OR) 5.22, Table 1, p = 0.01) and HCT-CI (OR 0.66 for increasing score, Table 1, p = 0.02. High-risk cytogenetics was not associated with exceptional responders (OR 0.43 yes vs no, p = 0.38) which may be due to low sample size.
We conclude that mel dosing impacts OS but not PFS in our cohort, though this effect is confounded by increasing age and potentially comorbidity. We report for the first time that mel200 dosing increases the rate of exceptional response compared to mel140. Overall, these data support recent conclusions by Sharma et al and challenge the previous reports of similar OS outcomes between mel200 and mel140 dosing.
Disclosures
Lunning:Regeneron: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Nurix: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Loxo: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria; InstilBio: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Fate Therapeutics: Consultancy, Honoraria; EUSA: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; CRISPR: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Acrotech: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; SeaGen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Curis: Research Funding. Vose:Eli Lilly and Company; Epizyme, Kite, Loxo, Novartis: Research Funding; AbbVie, MEI Pharma: Consultancy. Baljevic:BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen Biotech: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Cardinal Health: Consultancy; Parexel: Membership on an entity's Board of Directors or advisory committees. Holstein:Takeda: Consultancy; Sorrento: Consultancy; AbbVie: Consultancy; Genentech: Consultancy; GSK: Consultancy; BMS/Celgene: Research Funding; Oncopeptides: Consultancy, Research Funding; Janssen: Consultancy. D'Angelo:Abbvie: Consultancy; Seagen: Consultancy, Other: advisory board meeting attendence; Bristol Myers Squibb: Other: advisory committee meeting attendee x1, Research Funding; Fate Therapeutics: Research Funding; Ono Pharmaceuticals: Other: advisory board meeting attendee, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal